Introduction: Heparin-induced thrombocytopenia (HIT) is a serious adverse reaction to heparin. Antibodies against platelet factor 4 (PF4) or a heparin/PF4 complex have been reported to activate platelets, neutrophils and possibly endothelial cells, resulting in thrombocytopenia, hypercoagulability and thromboembolic complications. While the impact of anti-PF4/heparin antibodies on platelet, neutrophil and monocyte activation has been extensively studied, the role of endothelial cells in HIT-associated thrombosis remains underexplored. In particular, the interactions between HIT-induced procoagulancy and endothelial cells under dynamic conditions is not completely analyzed. In this report, we investigated how HIT antibodies induce thrombosis in an endothelialized microfluidic system.
Methods: Fibrinogen microfluidic channels were coated with confluent monolayer of human umbilical vein endothelial cells (HUVECs). Cells were primed with low-dose TNF-α to induce a sub-thrombotic endothelial activation, prior to perfusion of whole blood. Recalcified unstimulated or Thrombin Receptor Activator Peptide 6 (TRAP-6) activated whole blood was perfused at a venous flow rate. HIT-thrombosis model was established and tested with monoclonal anti-PF4/heparin antibodies in whole blood. In brief, whole blood was pre-incubated with unfractionated heparin (UFH, 0.2 IU/mL and 100 IU/mL). Next, anti-PF4/heparin antibodies were introduced to the whole blood mixture and incubated for 30 minutes. Whole blood was recalcified and perfused over resting or primed endothelial cells at a venous shear stress. Thrombus formation was recorded over time.
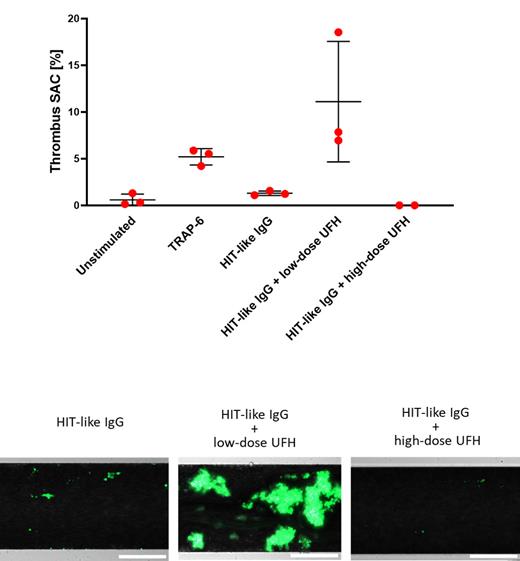
Results: The endothelialized microfluidic model successfully captures sub-thrombotic conditions under venous shear. Activated platelets induced a procoagulant shift and three-dimensional thrombi. We applied our thrombosis model to investigate thrombus formation induced by a HIT mimicking monoclonal antibody. We show that the heparin-dependent platelet activation predicts the thrombotic response in the microfluidic system: HIT antibodies in absence of heparin did not exert a pro-thrombotic effect on activated endothelial cells. Co-incubation with 0.2 IU/mL UFH induced thrombosis, embolization of thrombus material and channel occlusion only when endothelial cells were primed with TNF-α. The pro-thrombotic effect of HIT antibodies was fully reversed at the super-therapeutic dose of 100 IU/mL UFH.
Conclusions: HIT antibodies induce thrombosis and embolization in a system emulating physiological environment. For the first time, we present a comprehensive thrombosis model that incorporates the established thrombogenicity of HIT-mimicking antibodies. We show that our thrombosis model incorporates both endothelial- and blood-based control of thrombosis. Primed endothelial cells and antibody-induced procoagulancy trigger thrombosis in a concerted fashion, allowing investigation of the underlying mechanisms and possible preclinical evaluation of potential inhibitors of HIT-thrombosis.
Figure 1, upper panel: Maximal thrombus surface area coverage of TNF-α primed endothelial cells, perfused with unstimulated, TRAP-6 activated or HIT-like IgG/heparin challenged whole blood. HIT-like IgG did not increase thrombus formation in absence of heparin. Addition of low-dose heparin exerted a strong pro-thrombotic effect, that was fully reversible with a super-therapeutic concentration of heparin. Lower panel: representative images of thrombi formed by HIT-like IgG in absence and under low or high-dose heparin. Platelets: green, Calcein-AM. Scale bar: 200 µm.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal